Neuronal differentiation of human ACA-generated pluripotent stem cells and their potential application in cell replacement therapy US
Z.A. Becker-Kojić1,2, A. Schott1,2, I. Zipancić3, Herranz-Pérez V4, Hernández-Rabaza V5, J.R. Urena-Peralta2, M.P. Rubio2, M.G. Rosello2, M. Stojković2, J.M. Garcia-Verdugo42) Principe Felipe Research Centre, Valencia, Spain
3) Instituto de Ciencias Biomédicas, Facultad de Ciencias de la Salud, Universidad CEU Cardenal Herrera, Moncada, Valencia, Spain
4) Instituto Cavanilles de Biodiversidad y Biología Evolutiva, Universidad de Valencia, Valencia, Spain
5) NTBio, Biotechnology service company, Valencia, Spain
Introduction / Background
H uman neurological disorders are caused by a loss of neurons and glial cell in the brain. Cell replacement therapy to the diseased or injured brain should provide the basis for the development of new therapeutic strategies for treating many incurable neurologic disorders. Neural stem cells (NSC) transplantation could replace damaged tissue providing a cure effects. Pluripotent stem cells, embryonic stem cell (ESCs), induced pluripotent stem cells (iPSCs) or multipotent adult stem cells can be explored for tackling neuronal diseases. Each of these cell types has various disadvantages and contemporary, there is no efficient way to obtain autologous neural stem cells. Here, we report about human GPI-linked glycoprotein ACA a receptor involved in developmentally conserved signaling pathways.
Crosslinking of ACA initiates via PI3K/Akt/mTor a process of de-differentiation of blood progenitor cells lea- ding to generation of ACA pluripotent stem cells capable of differentiating into cell type of all three germ layers. Blood derived ACA pluripotent stem cells differentiate in vitro into neuronal cells with long branching structures which is confirmed by means of geno- and pheno-typical analysis. Above all, ACA cells differentiate in situ upon transplantati- on into cortex and striatum of immunocompromised mice into neural progenitor cells providing a pool of human NSCs which can be exploited for customized therapy of injured or diseased brain.
Generation and analysis of blood derived pluripotent stem cells.
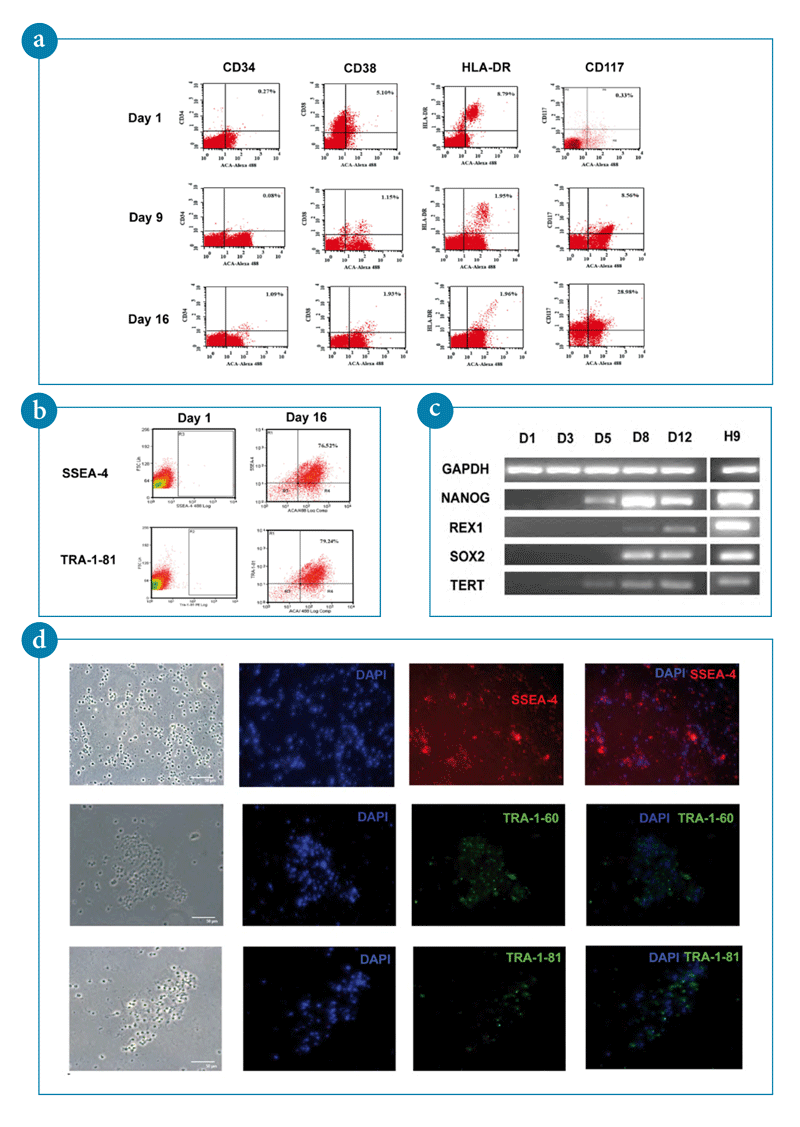
- A representative flow cytometry histogram showing de-differentiation of more mature blood progenitor cells in PBMNCs and generation of primitive ACA/c-Kit population not present in steady-state PBMNCs.
- FACS analysis of hESCs markers SSEA-4 and TRA-1-81 in pluripotent stem cells generated upon activation by ACA.
- RT-PCR analysis of the ES marker genes NANOG, REX1, SOX2, and TERT, in ACA-generated pluripotent stem cells.
- Immunostaining of pluripotency markers in ACA-generated stem cells grown on Matrigel coated plates. DAPI staining indicates the cell content per field. Images were taken by fluorescent microscopy.
In vitro differentiation potential of ACA-generated pluripotent stem cells into neuronal cells.
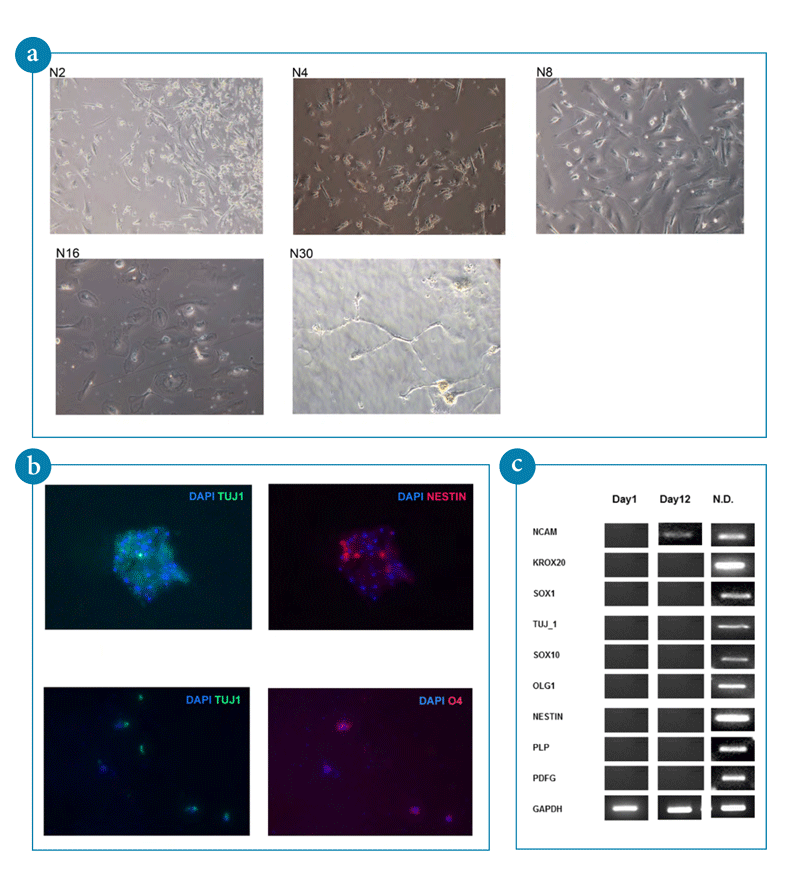
- Histomorphological images of ACA-generated pluripotent stem cells upon differentiation into neuronal cells. Images were taken by contrast phase microscopy..
- Immunostaining of typical neruonal markers (TUJ1, Nestin) and early oligodendrocyte marker (O4) in ACA-generated stem cells grown on ornithine/laminin-coated culture plates. DAPI staining indicates the cell content per field. Images were taken by fluorescent microscopy.
- RT-PCR analysis of genetic markers characterizing neuronal differentiation of ACA-generated pluripotent stem cells.
ACA-generated pluripotent stem cells differentiate in vivo into neural precursor cells
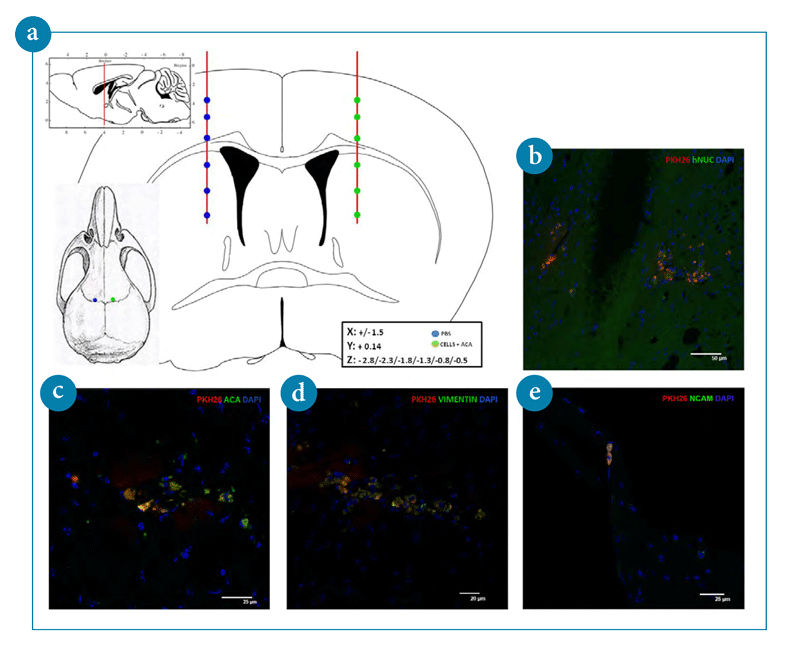
Immunohistology of frozen section of engrafted mice transplanted with PKH 26 labeled ACA-generated pluripotent stem cells. DAPI was used to visualize nuclear DNA.
- A schematic overview of in vivo transplanted ACA-generated pluripotent stem cells
- Human nuclei antibody is used to characterize engrafted human cells.
- Transplanted cells express human GPI-linked glycoprotein ACA.
- ACA pluripotent stem cells differentiate into neural precursor cells expressing vimentin.
- Engrafted ACA pluripotent stem cells differentiate into neural marker cell N-CAM.
Method
G eneration of human pluripotent stem cells was done by crosslinking of ACA at the membrane of immature blood cells with its specific GPI-antibody followed by incubation and de novo ACA-generated cells were grown in suspension in a culture plate with Iscove’s modified Dulbecco’s medium supplemented with 10% FBS, MEF feeder cells in Knockout- DMEM, or Matrigel in conditioned medium, res- pectively. Cells were taken at different time points for immunophenotyping by flow cytometry and immunofluorescence microscopy. The functionality of ACA-generated pluripotent stem cells was tested in vitro and in vivo through targeted differentiation in defined neuronal media, and xeno-transplantation assay using immunecompromised NOD/SCID mice.